What Actually Is a Heat Pump?


If you place a saucepan of water on the hob and turn on the hotplate, the water starts to heat up. If the hotplate is sufficiently hot, the water will also get hot and evaporate from about 100°C. If the saucepan is left without a lid, the steam that forms is also 100°C, and you can burn yourself on it. In a pressure cooker, both the water and the steam are hotter than 100°C.
A specific amount of heat is required to heat the water. This amount of heat is invoiced, as the amount of electricity on the electricity meter, for example. If heat is applied to the hot water, it eventually evaporates. For the evaporation, you need three times as much heat than you do to just heat the water.
If the steam leaves the saucepan and condenses on the extractor hood, for example, it releases heat again: at less than 100°C, the steam cools and turns into droplets of water; as a result of this, the extractor hood gets a little hotter. If the condensation were to be pumped back to the saucepan, it would create a closed circuit and a kind of heat pump. It’s that straightforward.
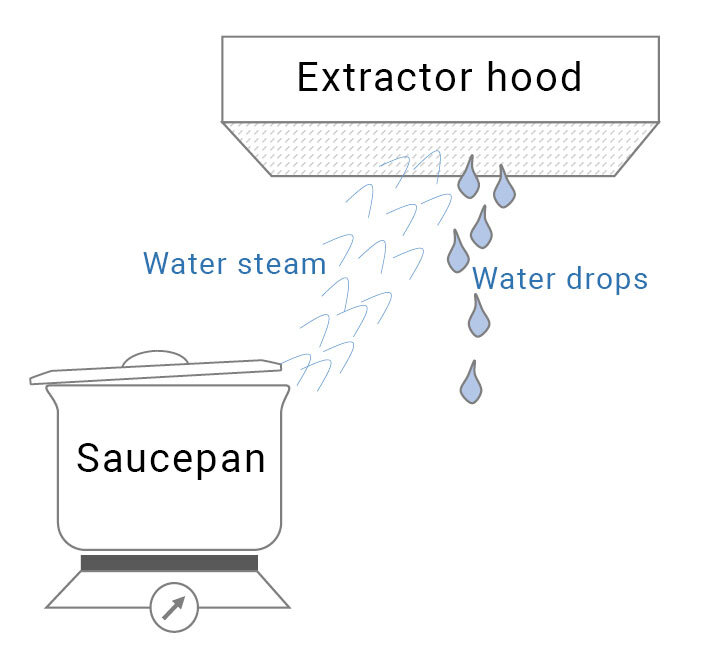
The advantage of the hotplate is that this is the way in which it gets hot. After all, heat only flows in one direction: from hot to cold. That’s the second law of thermodynamics.
And heat also flows from cold to even colder: a cold bottle of beer from the cellar can melt the ice, which is even colder, in the refrigerator. Therefore, remember the following: heat isn’t the same as temperature!
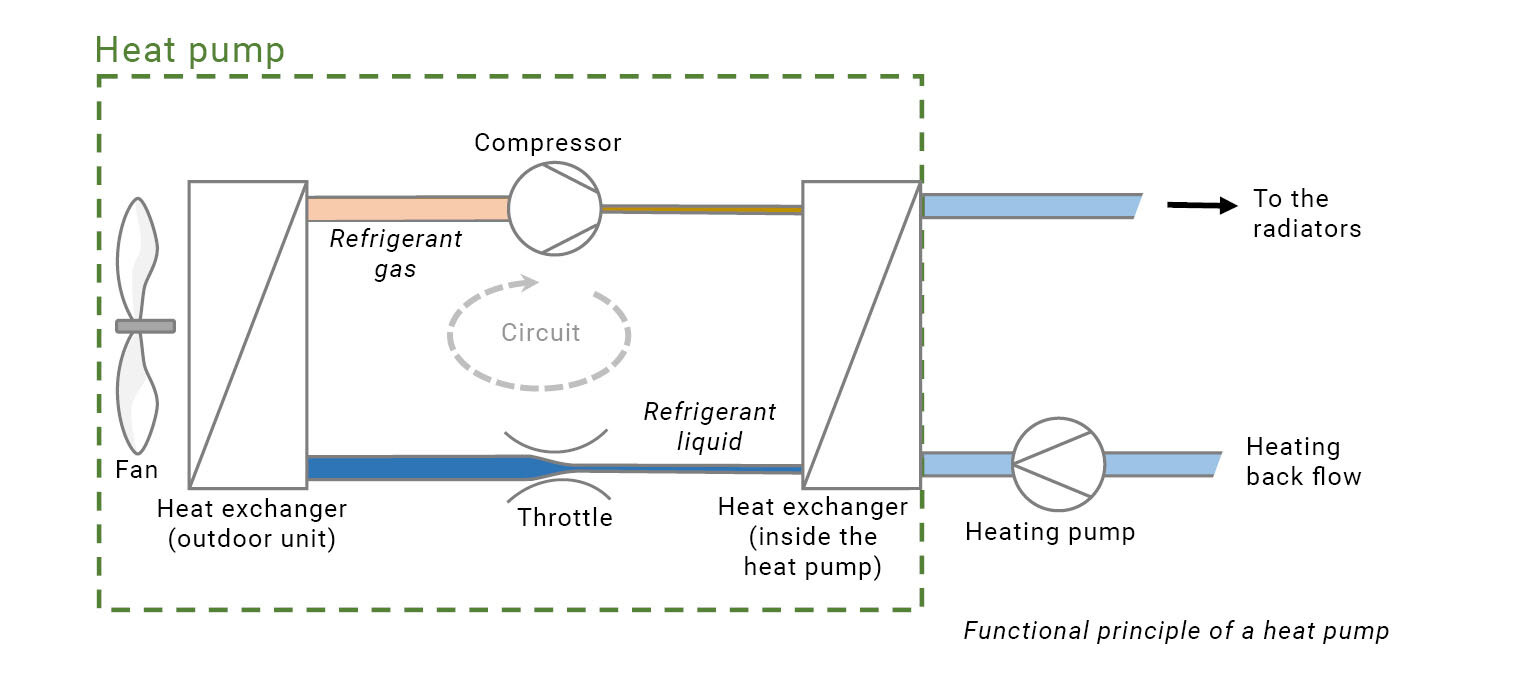
And this is where things get interesting from the technical perspective. Even if it’s -20°C outside, the heat which is stored in this cold air can make a difference: for example, it can cause dry ice which has a temperature of -78°C to evaporate. And that’s the very same principle of a modern heat pump: cold air also causes a refrigerant to evaporate at low temperatures.
The evaporated refrigerant is condensed again at another location, just like the water on the extractor hood. This was straightforward with the extractor hood, as it was colder than the steam from the cooker, and heat flows from a hotter to a colder state. Yet the refrigerant is cold, so how can someone heat their living room to 20°C? To do this, a technical trick is necessary: The condensation, and therefore the transfer of heat, works well as long as the pressure is changed as well. This is because if the pressure is increased quickly, the temperature increases. The refrigerant, in the form of a vapour, then condenses at approximately 20°C, and releases its heat at this higher temperature.
So that’s the complete principle of the heat pump and the refrigerator: 2 heat transfer surfaces, a pump to increase the pressure, and a “throttle” to reduce it. To start with, that’s all that’s necessary.
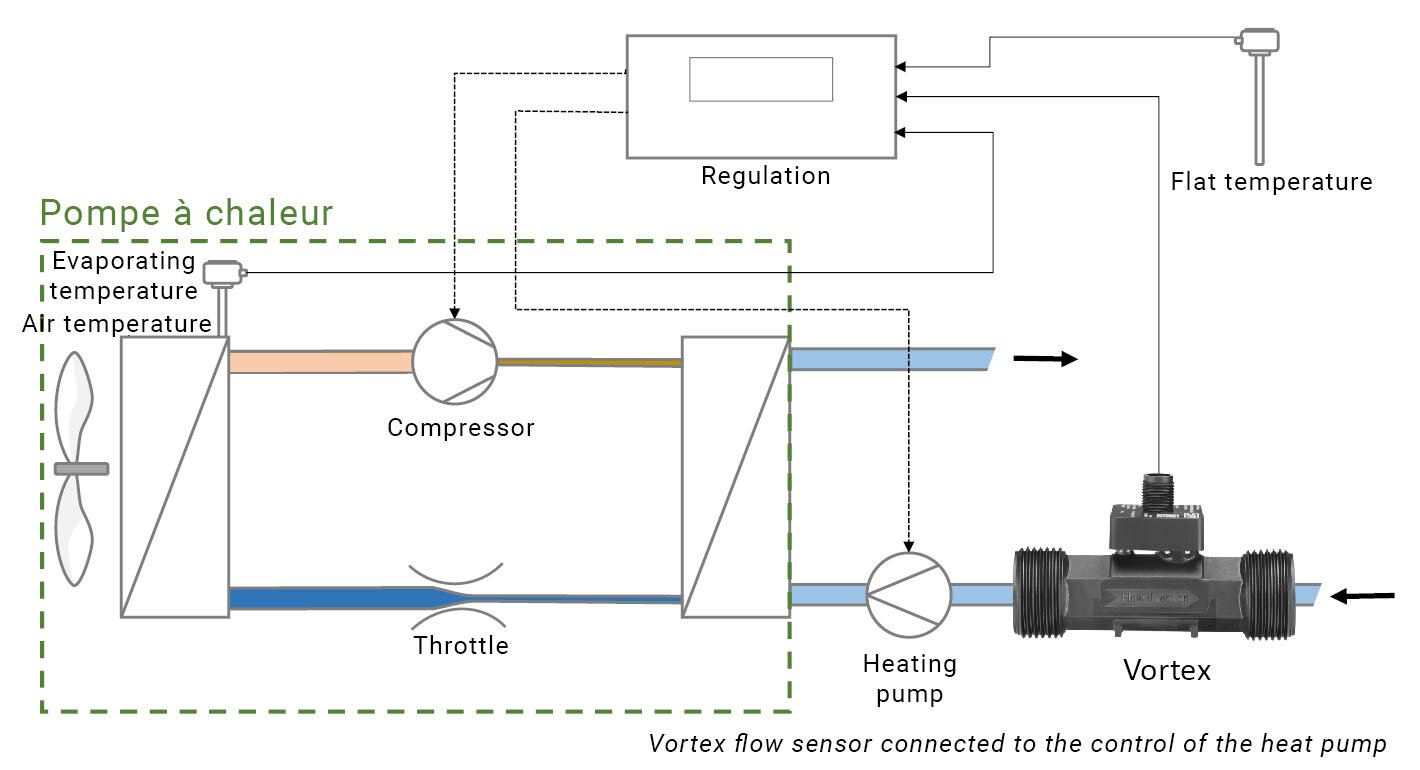
And to prevent the pumps from working away and consuming electricity, but to control the operation of the pump and prevent a possible freezing of the heat exchanger, the flow rate is measured using a SIKA Vortex flow sensor. In technical systems, there is also a reversible flow operation for defrosting the heat exchangers – this operating state also has to be controlled.